Special Authorization requirements for specialty drugs
As an additional step to help plan sponsors control and manage prescription drug costs under their plan, Saskatchewan Blue Cross is enhancing and expanding our special authorization requirements for a comprehensive list of high-cost, specialty drugs. These additional requirements will be implemented for new claimants effective October 1, 2024.
What is Special Authorization?
Special Authorization (SA) is a pre-approval process that helps us determine if certain prescription drugs will be reimbursed under a benefits plan, based on established medical criteria.
For coverage to be considered, additional information is needed to help us determine whether:
- the drug represents reasonable treatment for the condition;
- other or lower cost medications may be tried first to treat the condition;
- coverage is available under other provincial programs
How will plan participants know if a drug they are prescribed requires Special Authorization?
To check their drug coverage, they can log into the Group Member Portal or SK Blue Cross: Group app on or after October 1, 2024.
After entering the drug name or Drug Identification Number (DIN) into the Search Drug Coverage screen and selecting the appropriate DIN(s), they will receive one of the below coverage response messages:
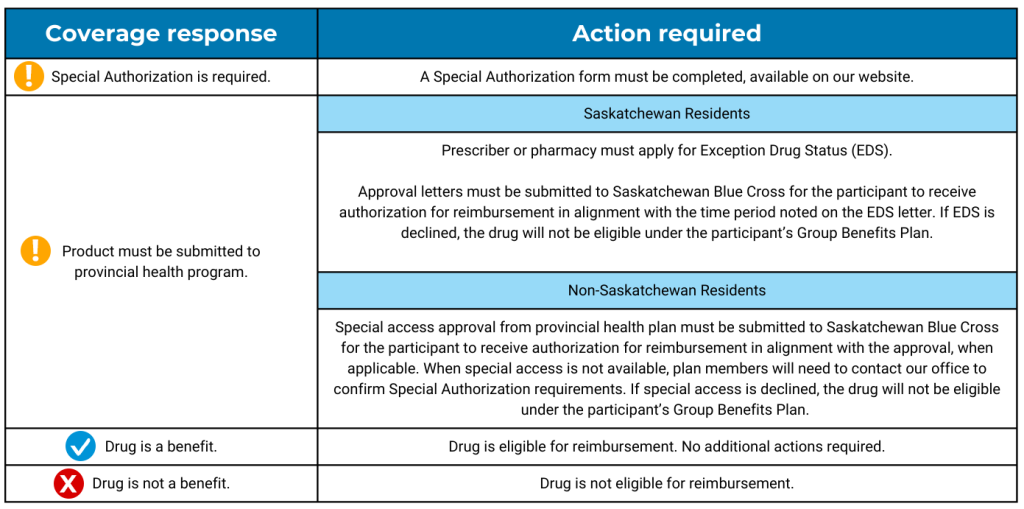
Forms & Documents – Group Benefits
Exception Drug Status | Extended Benefits and Drug Plan | Government of Saskatchewan.
If a plan participant is already receiving coverage for any of the drugs on our comprehensive Special Authorization list, they will not have to fill out a form and will not have any interruption in benefits.
How do plan members submit a Special Authorization form or Exception Drug Status (EDS) approval letter?
New claimants can submit to our office using one of the following options:
Email: providerrelations@sk.bluecross.ca
In-person: 516 2nd Avenue North, Saskatoon or 2275 Albert Street, Regina
Fax: 306.667.5860
Mail: Attn: Claims Department
Saskatchewan Blue Cross
516 2nd Avenue North, PO Box 4030
Saskatoon SK S7K 3T2
Alternatively, if a member has a claim to submit, the form or EDS approval letter can be attached with their submission via the member portal or mobile app.
Where do I find the Special Authorization eligibility criteria?
Eligibility criteria differs for each drug, is subject to change, and will not be posted publicly.
How long will it take for a Special Authorization form to be reviewed?
Once we receive a completed Special Authorization form, we will make every effort to process the request promptly and avoid delays. Plan members will receive a response by regular mail.
For certain Special Authorization drugs, coverage may only be approved for a specified time period and will be outlined in the confirmation letter. Should the drug be needed beyond the initial approval period, further information will be required for review.
How many drugs are on the Special Authorization drug listing?
Just over 2,000 Drug Identification Numbers (DINs) are currently on our list, comprised of drugs that are labeled with Exception Drug Status under the Saskatchewan Drug Plan and Extended Benefits Branch or are high-cost, specialty drug products that are found within our traditional, prescription-by-law plans; however, the list is fluid and subject to ongoing changes and updates.
